Mitochondrial membrane architecture in human health and disease
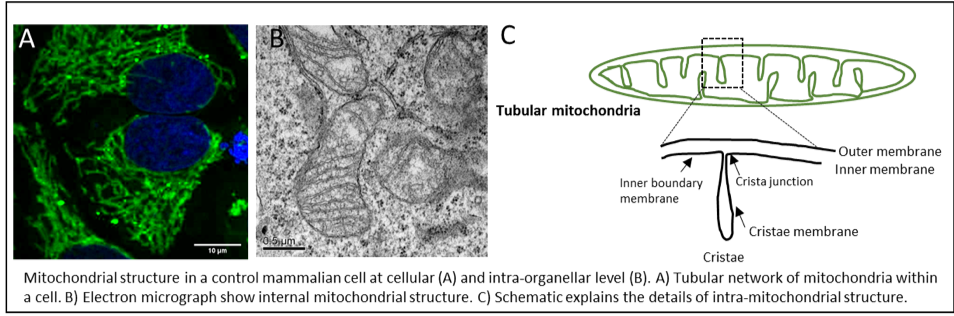
Research in my group is devoted to understanding how the structure of mitochondria is established and maintained during physiological and pathological conditions. For efficient cellular functioning, mitochondria have to constantly adapt to bioenergetic demands, metabolic changes and developmental cues. Invariably, mitochondrial structure changes dynamically at cellular as well as internal organellar level in response to these physiological cues. 1) In a cell, mitochondria appear as network of long tubes and short fragments that constantly undergo fusion and fission cycle to maintain the quality of the mitochondria. Large GTPases belonging to dynamin family are required for these processes, whose mutations occur in various human diseases. 2) Internal mitochondrial structure is also highly diverse and variable. Cristae size, shape or number changes based on physiological or energy demands. We recently show that cristae undergo cycles of fusion and fission within seconds (Kondadi, Anand et al. 2019). Cristae are separated from the rest of the inner membrane (called inner boundary membrane) by narrow opening called crista junctions. How are highly curved membrane-embedded crista junctions formed? How do they sense the energy status of cell? The major step forward in understanding the mechanism of cristae remodeling comes with identification of MICOS (mitochondrial contact site and cristae organizing system) complex. Mutations in subunits of MICOS, MIC60 and MIC13 leads to Parkinson’s disease or encephalopathy with liver dysfunction respectively. It becomes evident that maintenance of proper mitochondrial membrane architecture and deciphering the molecular basis thereof is important for understanding several human diseases, including neurodegeneration, cancer, diabetes or cardiomyopathy.
How are the changes in structure of mitochondria at cellular and organellar levels linked and sensed? We are focused to determine the molecular mechanism of the proteins responsible for these processes and how they influence the proper mitochondrial function. We aim to gain insights into the pathophysiological role of changes in mitochondrial architecture during human diseases. For our study we have generated several advanced tools and methods. For example, we use qualitative and quantitative imaging using electron and confocal microscopy for assessing mitochondrial structure. Various biochemical techniques including native gel electrophoresis, protein-protein interactions and analysis of mitochondrial function using a Seahorse Flux Analyzer are applied in our studies.



