Piekorz Group
1986-1992: Study of Biology at Friedrich-Alexander-Universität Erlangen-Nürnberg
1991-1992: Diploma thesis at Friedrich-Alexander-Universität Erlangen-Nürnberg (Characterization of Ca2+ and Calmodulin levels in primary hepatocytes and hepatoma cell lines)
1992-1997: Doctoral thesis (Dr. rer. nat.) at Friedrich-Alexander-Universität Erlangen-Nürnberg (Terminal differentiation of myeloid leukemia cell lines through IL6-type cytokines)
1997-2000: Postdoc at the Dept. of Biochemistry, St. Jude Children’s Research Hospital, Memphis, USA
2000-2002: Research Associate at the Howard Hughes Medical Institut, Dept. of Biochemistry, St. Jude Children’s Research Hospital, Memphis, USA
2002-dato: Senior Scientist, Institut für Biochemie und Molekularbiologie II, Medizinische Fakultät, HHU Düsseldorf
Publications: https://pubmed.ncbi.nlm.nih.gov/?term=piekorz+r&sort=date
Research
Sirtuins comprise a family NAD+-dependent enzymes that are involved in the regulation of various cellular aspects, including gene expression, metabolism, and proliferation (Fig. 1A). Our lab focuses on the biochemical and cell biological characterization of the primarily mitochondrially localized sirtuins, i.e., SIRT3, SIRT4, and SIRT5. Among them, we investigate the subcellular functions of SIRT4 in the regulation of mitochondrial dynamics, autophagy, and cellular proliferation. In this regard, SIRT4 is well characterized for its role as metabolic tumor suppressor in mitochondria. Increased levels of SIRT4, e.g., due to genotoxic stress in the cell cycle, inhibit the central metabolic enzymes pyruvate dehydrogenase (PDH) and glutamate dehydrogenase (GDH), thereby reducing ATP generation and proliferation. In addition to its mitochondrial role, SIRT4 localizes also extra-mitochondrially where it likely takes over further functions in the context of intracellular signaling and proliferation control. Here, recent findings of our group identified several cytosolically localized proteins as interaction/binding partners of SIRT4. In particular, SIRT4 binds to the RAF kinase paralog C-RAF/RAF1 thereby likely negatively regulating MAPK signaling (Fig. 1B). Our current work addresses the molecular basis of the SIRT4-C-RAF interaction and its significance for downstream signaling and cellular processes.
Recent articles from our group:
https://pubmed.ncbi.nlm.nih.gov/38499327/
https://pubmed.ncbi.nlm.nih.gov/37803520/
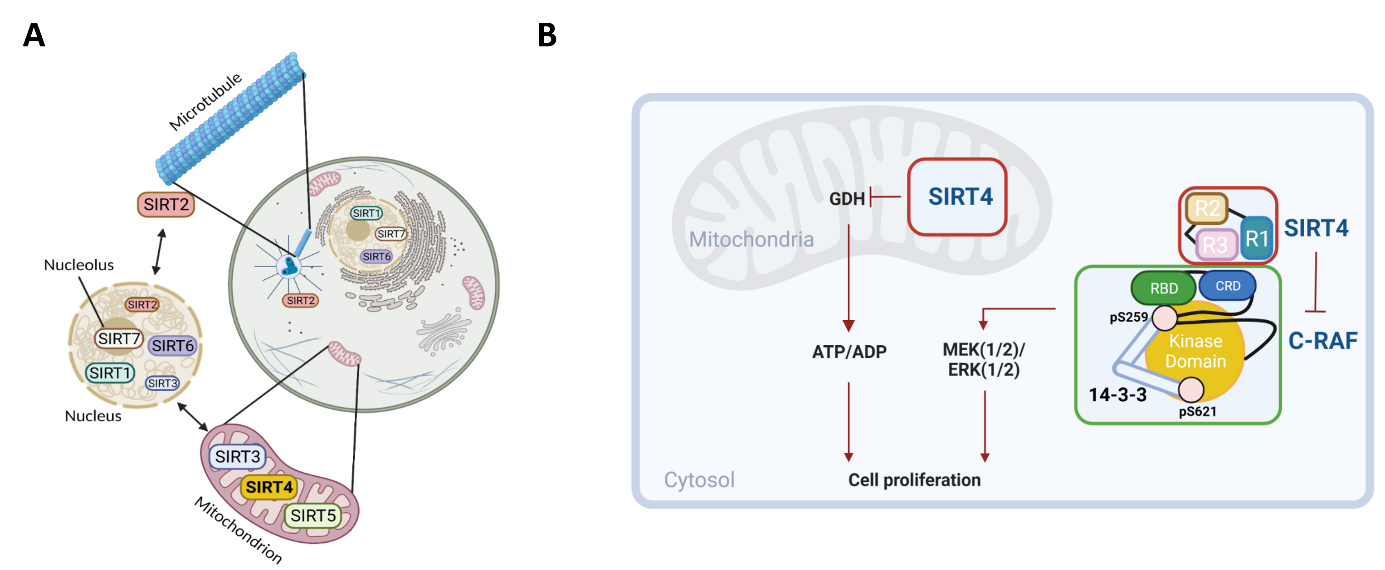
Fig. 1A: Cellular distributions and functions of human sirtuins. Figure by Dr. Laura Bergmann (created by BioRender).
Fig. 1B: Classical mitochondrial and novel extra-mitochondrial roles of the tumor suppressor SIRT4. The latter function is emphasized by the novel SIRT4-C-RAF interaction and subsequent regulation of the MAPK signaling pathway. Figure in B was taken from Mehrabipour et al., Life Science Alliance, 2024 (https://pubmed.ncbi.nlm.nih.gov/38499327/).


