PD Dr. Nina Graffmann, AG Liver Disease Modelling
In my group, we use induced pluripotent stem cells (iPSCs) to increase our knowledge about the human liver.
We therefore differentiate iPSCs towards hepatocyte like cells (HLCs) via a 3 step protocol. We have improved this protocol over the last years, to increase the maturity and functionality of the resulting hepatocyte like cells (HLCs) in 2D as well as in 3D. We then use the HLCs for liver disease modelling and want to establish them in the preclinical toxicity testing pipeline.
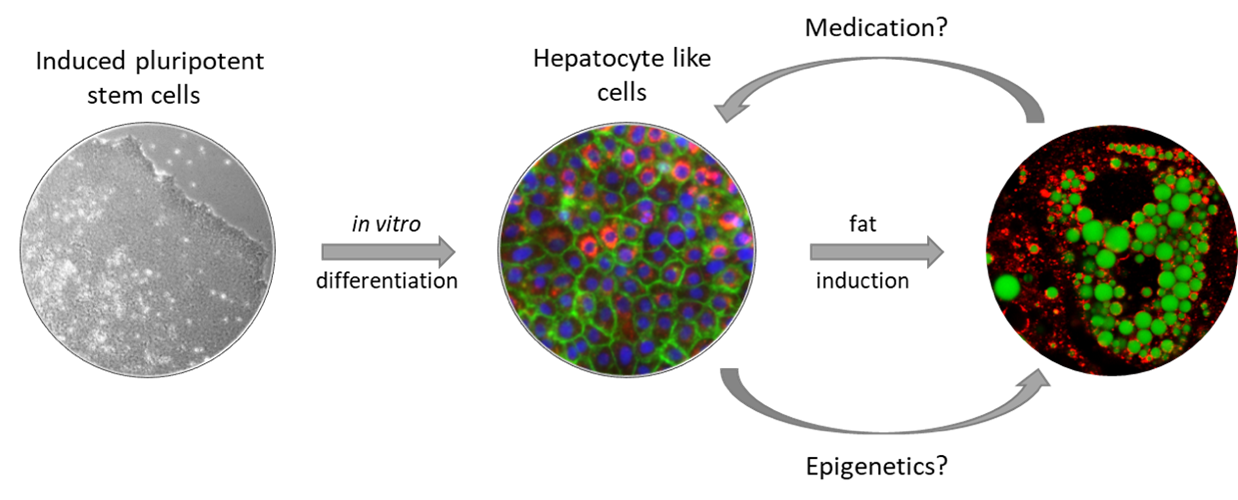
Fig. 1: Modelling of NAFLD with iPSC-derived hepatocyte like cells
Metabolic dysfunction-associated fatty liver disease (MAFLD).
In MAFLD (formerly known as non-alcoholic fatty liver disease (NAFLD)) the liver stores excess dietary fat as lipid droplets, which in the long turn impairs its function. The disease is getting increasingly prevalent in our modern world, affecting up to 30 % of the general population and 70 % of obese patients. Early MAFLD-stages are benign and reversible, however, 5-10% of the patients develop steatohepatitis, characterized by chronic inflammation, which can progress to fibrosis, cirrhosis, and hepatocellular carcinoma, finally necessitating liver transplantation. The phenotype is rather complex and the causes and mechanisms of disease progression are not fully understood yet.
In our in vitro model system, we can reproduce characteristic features of the disease such as fat storage in lipid droplets, increased expression of Perilipin 2 (PLIN2), and changes in the expression of metabolism associated genes in hepatocyte-like cells derived from donors with varying disease severity. We then use our cells to test various potential anti-MAFLD drugs which e.g. interfere with peroxisome proliferator-activated receptor (PPAR)α, adiponectin signalling, or dipeptidyl-peptidase 4 (DPP4) activity. To better understand the role of DPP4 in the development of liver fibrosis and fibrotic diseases in other organs, we are currently working on a CRISPR-based DPP4 knockout iPSC line.
Until now, we could only model early stages of MAFLD and we are working on more complex (3D) models comprising additional cell types to also reproduce fibrosis in the cell culture.
Alpha-1-Antitrypsin Deficiency (AATD)
AATD is a rare disease, which is primarily known for its lung phenotype, namely emphysema and COPD. It develops usually in higher ages as a result of chronic increased inflammation in the lung. AAT is produced and secreted by hepatocytes. In healthy individuals, it is transported to the lungs, where it attenuates the inflammation promoting molecule neutrophil elastase. However, in patients harbouring a missense mutation called Z-allele, it polymerizes inside the hepatocytes, preventing its secretion and causing liver damage such as fibrosis and cirrhosis. While many patients are only diagnosed later in life, sometimes liver symptoms occur already in early childhood. Children suffer mainly from cholestasis, which in its severe form can destroy the liver and necessitate liver transplantation. Overall, AATD accounts for about 3% of all paediatric liver transplantations.
In cooperation with researchers from the University Clinic Aachen and Bonn, we want to understand why there are two distinct phases in life when patients develop a liver phenotype and why some do not develop liver problems at all.
As there is no approved treatment for the AATD associated liver phenotype, yet, we also want to use our model for testing novel potential drugs.
Toxicology
As the main metabolic organ of the body, the liver is responsible for metabolising xenobiotics. While the main goal here is to de-toxify them and enable excretion via urine or bile, some substances become activated and gain toxicity after passing the liver. This is an important factor that needs to be considered during the development of drugs. We aim to improve our HLC culture and functionality in order to provide an in vitro system for testing the effects of liver metabolism on new compounds and replace animal experiments in the long run.
Funding: Christiane + Claudia Hempel Stiftung für regenerative Medizin
Team
PD Dr. rer. nat. Nina Graffmann
M.Sc. Christiane Lörch


