Research focus
Investigation of protein misassembly, misfolding or misprocessing in brain diseases, and the development of pharmacotherapies to interfer with or prevent these protein pathologies. There is a focus on neurobiology and proteinbiochemistry. Generation of (mis)assembled proteins is investigated at the levels of generation, recognition and degradation in in vitro and in vivo models.
1. Identification of external trigger factors and their cellular interfaces for diseases involving protein aggregation of misassembly. Specifically, we use viruses (influenza virus; herpes virus and SARSCoV2) to decipher cellular circuits involved in triggering protein aggregation and misassembly and develop drugs to prevent this (Müller-Schiffmann et al.).
2. Neurobiology of chronic mental illnesses (CMI) like schizophrenia and recurrent affective disorders. Identification and characterization of CMI-associated protein pathology by proteomics, biochemistry, cell biology. Characterizing sporadic CMI as protein misassembly disorders, starting with but not limited to the Disrupted-in-Schizphrenia 1 protein (Bader et al). Development of animal models for CMI (Trossbach et al.).
3. Translational research for redefining chronic mental illnesses by their underlying biology / biomarkers. Discovery and validation of biological markers as diagnostic tests for schizophrenia. See our review on blood tests for schizophrenia in Lancet Psychiatry (Korth & Fangerau).
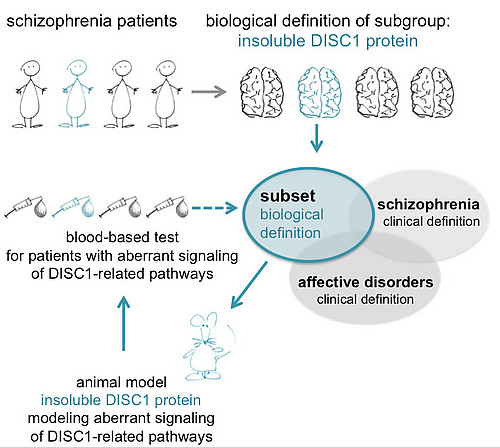
We recently identified an immune biotype for a subset of schizophrenia with potential for a future diagnostic blood test (Trossbach et al. see accompanying videoclip here). Further neuroimmunological signatures in schizophrenia subsets are currently characterized.
4. Cell biology and functional regulation of protein conformers. Generation of conformation-sensitive ligands, monoclonal antibodies and recombinant antibodies for diagnosing and characterizing protein conformational diseases, in particular prion-protein associated diseases and Alzheimer's disease. Development of animal models favoring specific protein conformers: see here a video on our tgDimer mouse that expresses exclusively Abeta dimers and is a model for early Alzheimer’s disease (Müller-Schiffmann et al.; Abdel-Hafiz et al.).
5. Neurobiology of Aging. Insoluble and aggregated protein deposits are not only hallmarks of neurodegenerative disorders, but are also hallmarks of the aging brain in the form of accumulating lipofuscin and other cellular debris. Dysfunctional protein degradation in the aging post-mitotic cell is investigated by proteomic, biochemical and cell biological techniques and correlated to cognitive dysfunction. Cross-influences to protein conformational diseases are revealed.


