Research focus
Brain tumors are highly addicted to glucose to develop and expand. However, due to high glucose consumption and poor nutrient supply within the tumor microenvironment, brain tumors are subjected to glucose-deprived conditions. These clearly limit their capacity to synthesize biomolecules and produce energy, in turn impeding their growth. In addition, activation of oncogenes, such as MYC, RAS and AKT, is known to render cancer cells more sensitive to glucose depletion. This highlights that at early stages of tumorigenesis, brain cancer cells are particularly vulnerable to the glucose-limited conditions of the tumor microenvironment, and therefore need to adapt in order to develop as macroscopic tumor masses. Inadvertely, the adaptation of brain cancer cells leads to the emergence of more aggressive tumor cells, such as tumor initiating cells which resemble cancer stem cells. A major question is which mechanisms underlie brain tumors adaptation to glucose deprivation? Delineating such mechanisms is critical not only to better understand brain cancer biology, but also to provide novel therapeutic targets.
We propose that brain tumors hijack endogenous pathways, which are conserved in the evolution and which protect normal tissues against glucose deprivation. We believe that if we understand how normal cells/tissues respond to these conditions, we will learn how tumor cells/tissues adapt to these conditions.
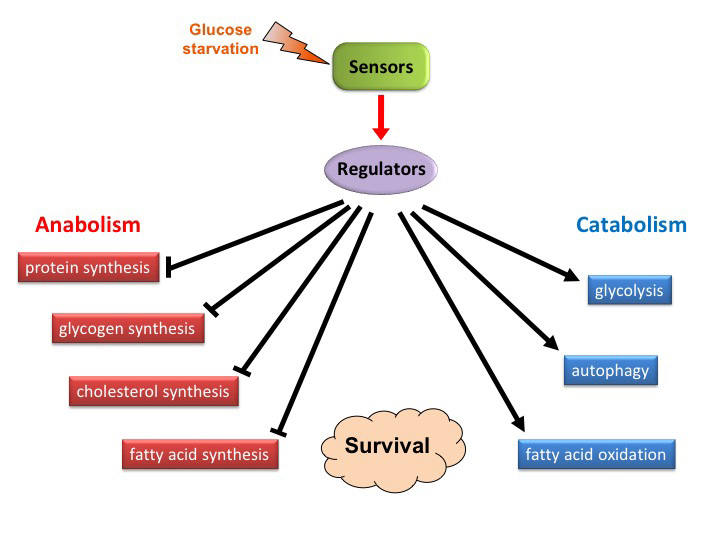
In normal cells, there is a very well-coordinated intracellular response to glucose deprivation. Key sensors respond to any changes in glucose levels, in turn modulating the activity of specific regulators through signaling cascades. These regulators induce a metabolic switch, where a number of anabolic processes (protein synthesis, fatty acid synthesis,…) are being blocked, to prevent energy consumption, while a number of catabolic processes (fatty acid oxidation, autophagy,…) are being activated, to generate energy and antioxidants. Such a metabolic reprogramming is critical to preserve cell viability under low glucose conditions. Our previous work highlights that a signaling pathway that restrict protein synthesis and which is conserved in the evolution, the AMPK-eEF2K pathway, is critical to protect normal and cancer cells under glucose-deprived conditions. The importance of other regulators of protein synthesis are currently being investigated in brain cancers. This promises to uncover novel players of brain cancer stress resistance, which can be targeted therapeutically.