Molecular Medicine – Projects
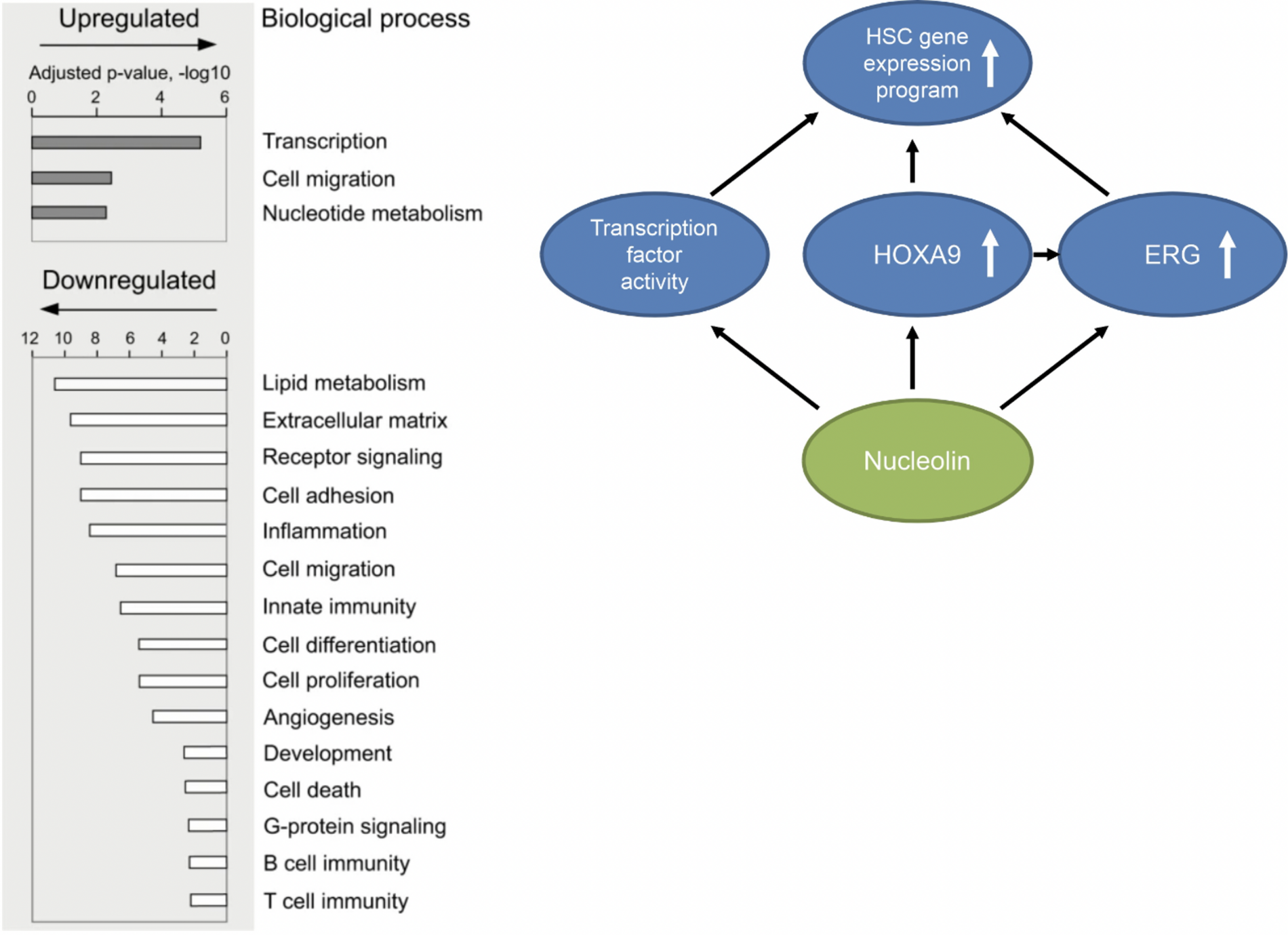
Signal Transduction and Gene Regualtion in Hematopoietic Stem Cells (HSCs) - The research group Grinstein focuses on the processes of normal and aberrant signal transduction and gene regulation in hematopoietic stem and progenitor cells (HSPCs), using methods of cell and molecular biology and hematology.
Hematopoietic stem cells are capable of self-renewal and differentiation to replenish blood cell lineages. Hematopoietic stem cell transplantation is a stem cell therapy successfully used for decades, and ~50.000 patients undergo hematopoietic stem cell transplantation annually worldwide.
Our research aims at gaining new insights into the hematopoietic stem cell compartment at molecular, intracellular and intercellular levels, to improve understanding of these cells and their therapeutic application.