Hepatoimmunology Group
Research
The liver from an immunological point of view.
Beside its crucial relevance for metabolism, detoxification and the excretion of waste products, the liver also plays an important role in immune regulation. It produces acute-phase proteins that are involved in controlling inflammatory responses, supporting pathogen defense and promoting tissue repair. Furthermore, it contains the largest population of tissue-resident macrophages in the body, which are immune cells essential for maintaining homeostasis and tissue regeneration, as well as recognizing threats and participating in the orchestration of subsequent immune responses.
The Hepatoimmunology Group's research focuses on the molecular signaling networks that regulate immunological responses at the intra- and intercellular levels. A particular focus is the mutual influence of immune and parenchymal cells in the liver, and their impact on inflammatory reactions and immune responses. This includes investigating how metabolic stress, inflammation and infection alter communication between different liver cell types, thereby promoting or modulating the development of chronic liver disease. It also involves studying how these intra- and intercellular communication processes and their disruption affect systemic immune function.
Two main areas are currently under investigation:
1. Cell-to-Cell Communication in Liver Immunity and Disease
This research addresses the question how signals from cytokines and pathogens are processed by functional (parenchymal) and structural (non-parenchymal) liver cells, including immune-related cells. Particular attention is given to the effects of these signals and the intra- and intercellular signaling networks they influence on acute-phase responses, liver regeneration, and the development of metabolic liver diseases.
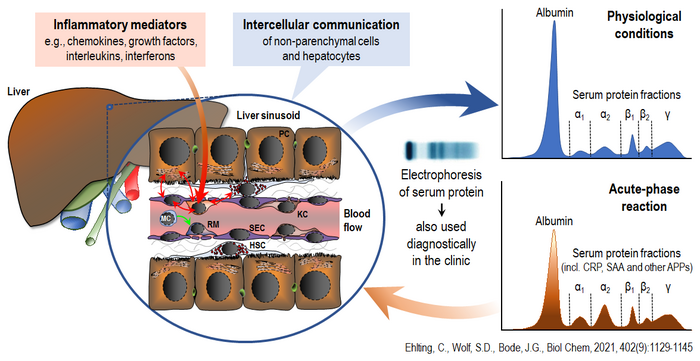
In this context, signaling pathways involving MAP kinases and the JAK/STAT cascade are being investigated for their role in regulating the synthesis of acute-phase proteins. These proteins are released by the liver according to the ‘sprinkler principle’ (Figure 1). It is not yet known how this process can be fine-tuned and, where applicable, optimized in response to inflammatory challenges.
Moreover, recent studies from our group have demonstrated that the activation state of liver macrophages is largely determined by their cellular origin under specific conditions. This is evident from the observation that, even under homeostatic conditions, macrophages derived from monocytes recruited from the circulation are influenced by their proximity to hepatocytes, the liver's primary parenchymal cells. In a hepatocyte-dominated microenvironment, these macrophages adopt a distinct activation state via a mechanism involving downregulation of the type II receptor for the signaling molecule TGF-β (Figure 2). Disruption of hepatocyte function, for instance, through metabolic dysregulation, can therefore alter macrophage behavior, impairing their ability to respond to injury or infection.
As part of the BMBF-funded ‘LiSyM Cancer’ research network, the working group is using a systems biology research approach to characterize the activation state of tumor-associated macrophages that become detectable during the development of hepatocellular carcinoma and to determine the extent to which these are derived from a defined macrophage subpopulation of the liver.
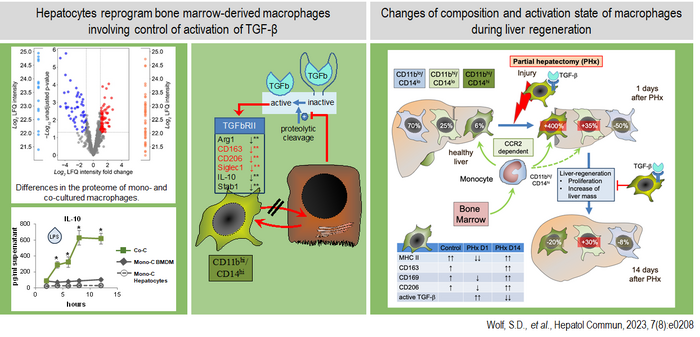
Left part: Hepatocytes strongly influence the activation state of CCR2-dependent recruited macrophages in co-culture by inhibiting the activation of latent TGF-β. Under homeostatic conditions, hepatocytes induce a more anti-inflammatory phenotype in CD11bhigh and CD14high expressing macrophages, characterized by increased expression of IL-10, CD163, and CD206. Right part: Following liver injury, the composition of hepatic macrophages shifts rapidly. The number of Kupffer cells, originating from embryonic precursors, decreases, while CCR2-dependent recruited macrophages increase significantly within one day. During this early phase, there is a strong activation of TGF-β, leading to elevated expression of pro-inflammatory cytokines by the recruited macrophage population, which in turn influences the course of liver regeneration.
2. Interference of viral pathogens causing persisting infections with host- and immune-cell function.
Viruses that persist in the body over time, such as hepatitis C virus (HCV) and cytomegalovirus (CMV), have evolved sophisticated mechanisms to evade immune defenses while maintaining host cell viability. These viruses are of particular interest because they alter liver cell behavior by hijacking intra- and intercellular signaling pathways, thereby reshaping the immune microenvironment to their advantage.
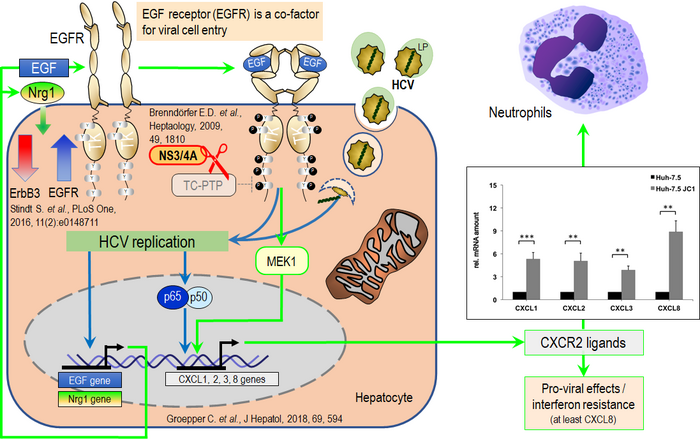
HCV, for example, has been shown to reprogram the signaling networks of its host cell, thereby modulating cellular responses to various stimuli and the inflammatory milieu, ultimately promoting viral replication. Our investigations reveal that HCV disrupts innate immune mechanisms in the host cell via a virus-encoded protease that cleaves key regulatory proteins. Additionally, it interferes with the ubiquitously expressed ‘T-cell protein tyrosine phosphatase’ (TCPTP), a crucial endogenous negative regulator of ‘epidermal growth factor receptor’ (EGFR) signaling. Studies have shown that this receptor plays a key role in the entry and replication of viruses into host cells. Consistently, HCV also enhances the surface expression of EGFR by modifying the expression of other members of the ErbB receptor family. Together with additional viral strategies that interfere with host cell factors, these mechanisms substantially alter host cell responses to external stimuli. Further elucidation of this phenomenon is a key focus of our research, particularly because it affects the expression and secretion of chemotactic signals by the host cell, thereby profoundly shaping the liver’s inflammatory and immune environment (Figure 3).
CMV infection elicits host cytokine responses, most notably the immunomodulatory production of IL-10 via IFNAR1-mediated signaling. Our findings reveal that this process critically depends on the intracellular kinase MK2. This also restricts the formation of ‘intrahepatic myeloid-cell aggregates of T cell population expansion’ (iMATEs), suggesting a regulatory function in preventing excessive immune cell clustering within the liver. Understanding the physiological consequences of this pathway remains a key scientific interest.
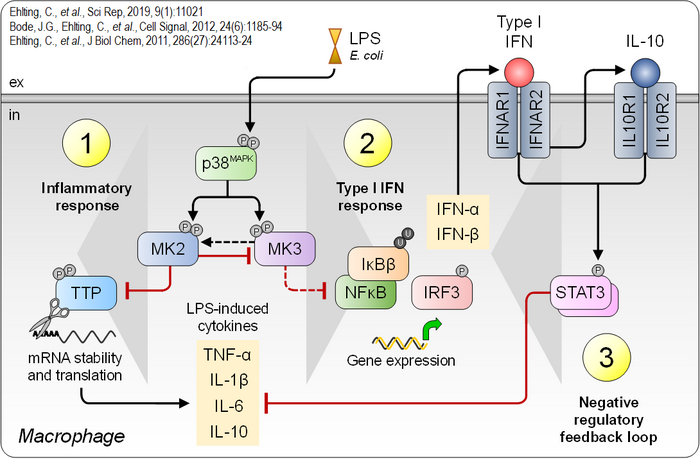
A central molecular focus in both research areas is the p38MAPK signaling pathway and its downstream effector kinases MK2 and MK3. This pathway regulates the production of a wide range of cytokines and is therefore critically involved in innate and adaptive immunity, as well as in tissue damage response and repair mechanisms. Findings from our group suggest that p38MAPK-MK2/3 constitute a complex signaling hub, in which MK2 and MK3 modulate each other's function in a context- and likely cell type-dependent manner. Studies in macrophages exposed to bacterial components such as lipopolysaccharide (LPS) demonstrate that this module plays a pivotal role in the initiation, propagation, and resolution of inflammatory responses (Figure 4). Consequently, targeting this signaling pathway offers promising strategies for regulating excessive inflammatory reactions.
Understanding, how the intra- and intercellular networks and the different types of liver cells are engaged in control and regulation of immunity and inflammatory responses, and how these processes are dysregulated in disease, offers valuable insights into new therapeutic approaches for liver conditions and chronic infections.