Coaxial Bioprinting of Blood Vessel Grafts
In the modern world, vascular pathologies are at the root of an ever-increasing number of disease cases. Chronic atherosclerotic changes in the vasculature can lead to the occlusion of blood vessels, restricting blood flow and impeding proper organ perfusion. This issue is most prominently observed in coronary artery disease, where a coronary artery is stenotic to the extent of decreased or complete cessation of blood flow to a portion of the myocardium. These situations often require surgical intervention and the establishment of a bypass circuit. Typically, a blood vessel from the patient is harvested and used to bypass the stenotic part of the coronary artery, reestablishing proper blood flow to the affected myocardial tissue.
However, the availability of adequate autologous grafts in patients is oftentimes limited by degenerative processes that deteriorate the patient's vasculature due to comorbidities such as diabetes mellitus, atherosclerosis, or chronic venous insufficiency. Additionally, previous blood vessel harvests or traumatic tissue injuries can make further explantations impossible. Moreover, the removal of autologous blood vessels is associated with impaired wound healing at the harvest site and increased patient distress.
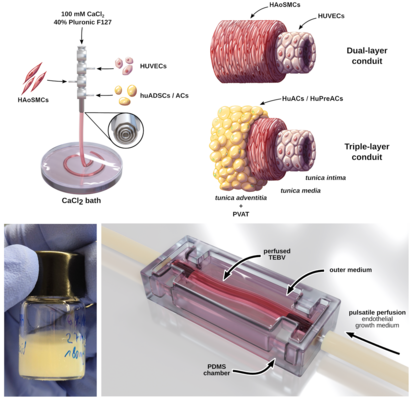
Our group aims to develop a novel platform for the on-demand generation of patient-specific, 3D-printed blood vessels, tailored to meet individual patient needs. Utilizing coaxial bioprinting technology, we strive to create multilayered conduits that mimic the exact architecture and spatial distribution of cells found in native blood vessels. Our specially formulated bioinks will provide a robust hydrogel scaffold and all the essential biochemical cues for optimal cell adaptation to the vascular environment. Through pulsatile perfusion in a customized bioreactor, our bioengineered blood vessels are further conditioned and allowed to mature into fully functional grafts.
Beyond vascular surgery, we are also exploring the burgeoning field of artificial organ fabrication. Our research seeks to contribute to this effort by developing a platform capable of generating perfusable vascular networks around which corresponding organs can be assembled. Currently, the creation of macro-scale artificial organs is constrained by the inability to provide adequate vascular perfusion, leading to the premature suffocation of deep tissues. We aim to address this challenge with our bioprinting platform, providing the necessary vascular infrastructure to support the development and viability of artificial organs.




